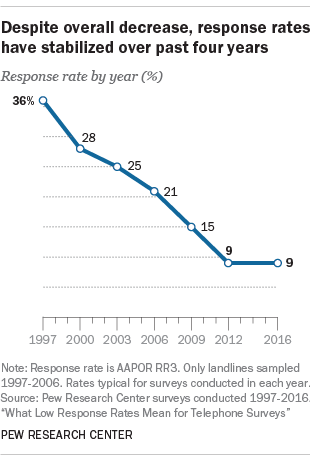
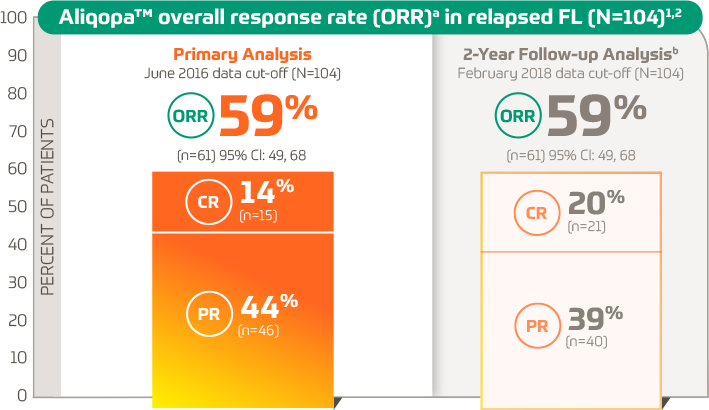
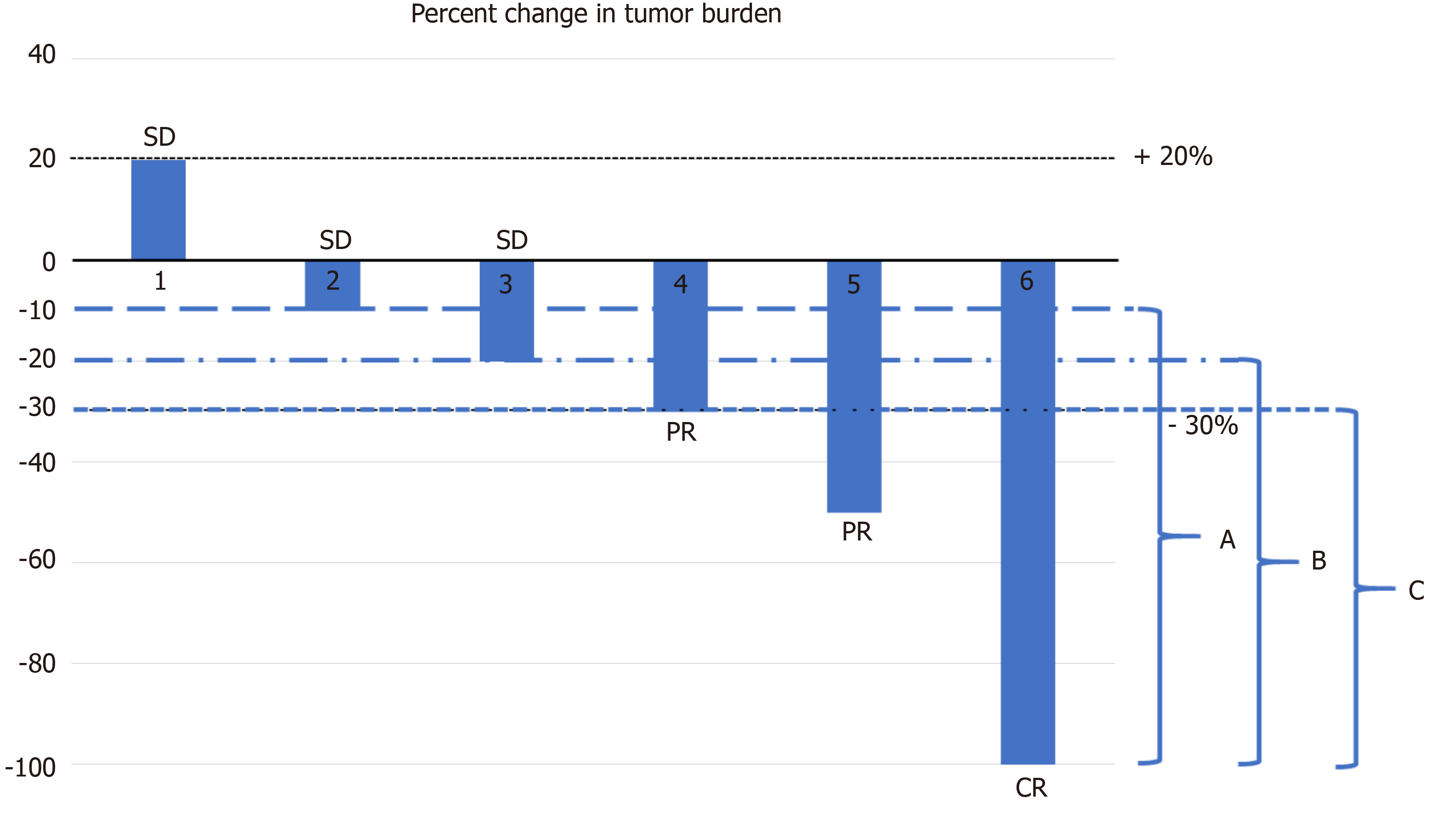
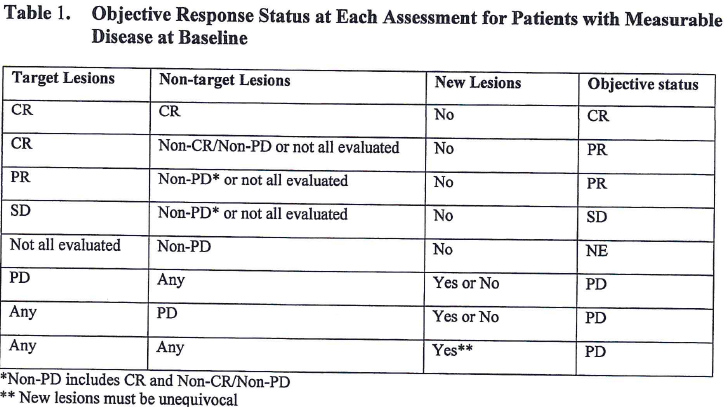
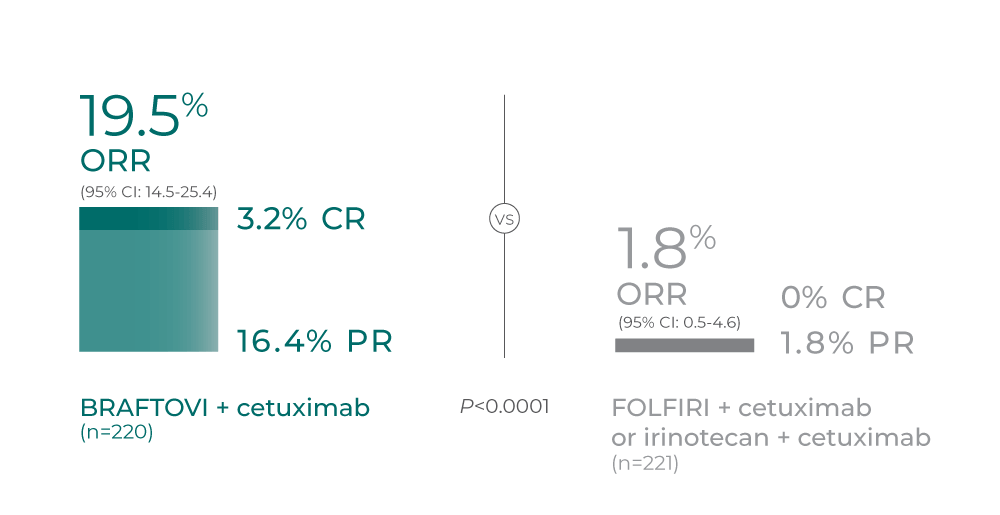
Response rates Overall response rate and depth of response according to... | Download Scientific Diagram
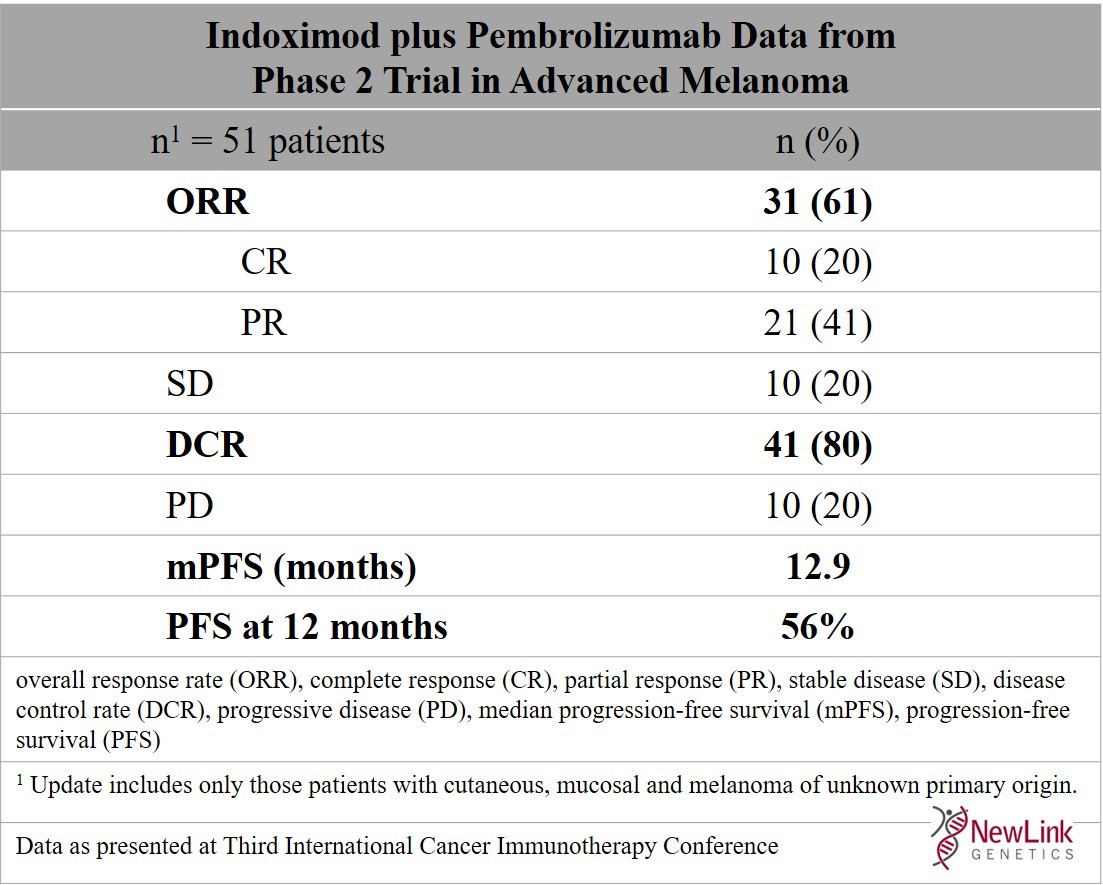
Updated Data for Indoximod Plus KEYTRUDA® (pembrolizumab) Demonstrate Improvement of Response Rate for Patients with Advanced Melanoma | Business Wire

Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial | Nature Medicine
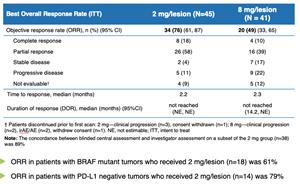
Overall Response Rate of 76% in Advanced Melanoma Patients with Dynavax's SD-101 in Combination with KEYTRUDA® (pembrolizumab); Data Presented Today at the 2019 ASCO Annual Meeting | Dynavax Technologies Corporation
Nexcella Announces Positive 58-Patient NXC- 201 Clinical Data: 100% Overall Response Rate in light chain (AL) Amyloidosis; 92% Overall Response Rate in Multiple Myeloma at the EBMT 49th Annual Meeting in Paris | BioSpace